Battery and bulbs are like salt and pepper in every household. We can never have enough of them! Whether it’s choosing a battery for a car or buying a bulb for your kitchen, these purchases are never-ending. What if you could power your single-use electronics with paper batteries that are not only good for the environment but also easy to use?
Empa researchers have created a disposable paper battery that is triggered by water. It has the potential to power a wide range of low-power, single-use electronic devices, such as smart tags and environmental sensors.
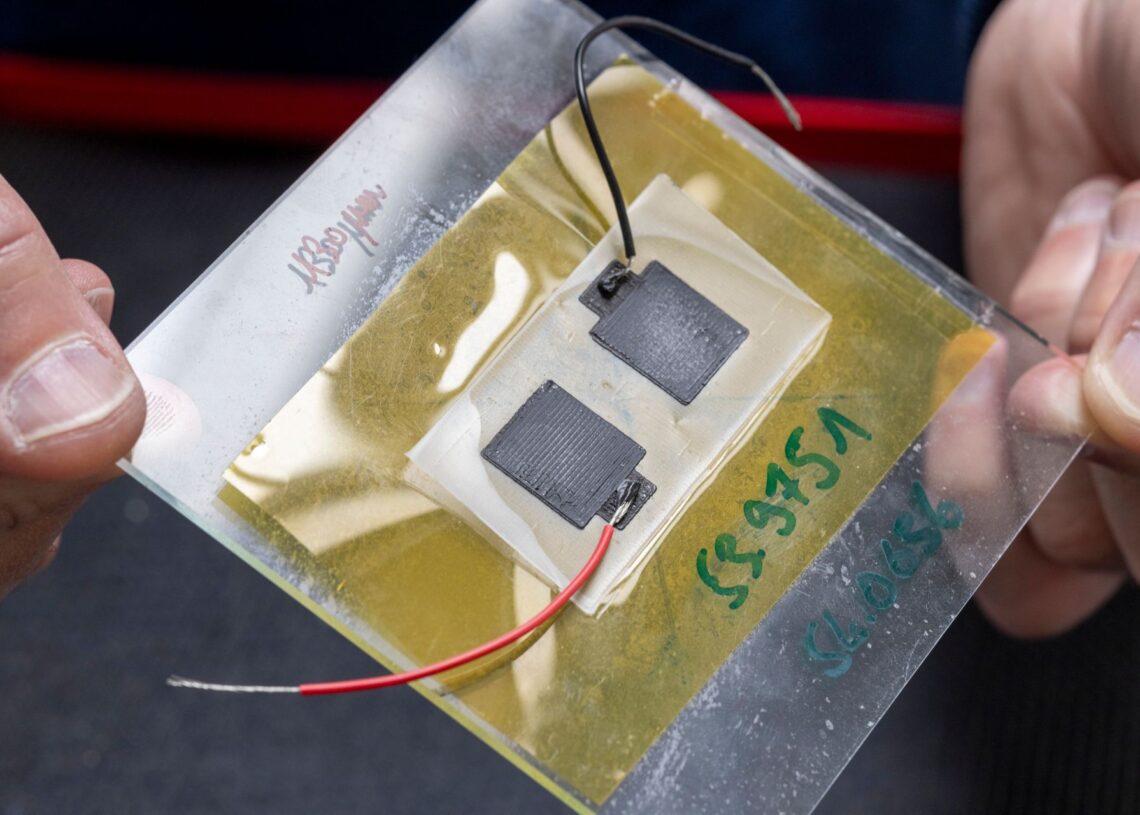
Gustav Nyström and his team designed the battery, which comprises at least one cell of one square centimeter and is made up of three inks printed on a rectangular strip of paper. According to the Federal Laboratory for Materials Testing and Research (Empa), table salt is sprinkled on the strip of paper, one end of which has been dipped in wax.
How Does it Work?
Simply adding water to activate the paper battery is the solution. It is environmentally friendly because it is biodegradable and readily thrown away. The battery is designed for such single-use electronics to minimize their environmental impact.
On one of the flat sides of the paper, graphite flakes are printed to represent the positive end of the battery (cathode), while zinc powder is printed to represent the negative end of the battery (anode).
On top of the other two inks, another ink containing graphite particles and carbon black is printed on both sides of the paper. This ink serves as the current collector, connecting the positive and negative poles of the battery to two wires positioned at the wax-covered end of the paper.
When a small amount of water is introduced, the salts in the paper dissolve, and charged ions are liberated, resulting in a conductive electrolyte.
These ions activate the battery by scattering in the paper, causing the zinc in the ink at the anode to oxidize and release electrons.
The authors merged two cells into one battery and used it to power an LCD alarm clock to demonstrate the battery's potential to run low-power gadgets.
Performance
Analysis of the performance of a one-cell battery found that when two drops of water were added, the battery activated within 20 seconds and attained a stable voltage of 1.2 volts when not connected to energy-consuming equipment.
A standard AA alkaline battery has a voltage of 1.5 volts. Due to the drying of the paper, the performance of the one-cell battery fell dramatically after one hour. However, after adding two more drops of water, it maintained a consistent operating voltage of 0.5 volts for more than an hour.
The scientists discovered that the amount of zinc in the paper has a substantial impact on the capacity of the paper battery, which may be tweaked for different purposes. With the proper techniques, both zinc and graphite are biodegradable and recyclable, lending credence to the paper battery as an environmentally safe power storage technology.
Looking for more battery-related products? Check out these battery chargers and battery testers.
We hope you love our reviews! For your information, we do earn money from commission in the link in the content! For more information click here!














